Reduce Ear Buzzing Using This Method
Latest Advances in Tinnitus Oto 313 Research and Relief

Understanding Tinnitus and the Quest for Relief
What is Tinnitus?
Tinnitus is a common auditory condition characterized by the perception of sound without an external source. Individuals with tinnitus often describe it as a ringing, buzzing, or hissing in the ears. It can be intermittent or constant, and its intensity varies from a mild annoyance to a debilitating condition. The causes of tinnitus are diverse, including hearing loss, exposure to loud noise, earwax blockage, and certain medications. Addressing tinnitus requires a deep understanding of its multifaceted nature and the pursuit of innovative treatments.
The Impact of Tinnitus on Quality of Life
The impact of tinnitus on quality of life can be profound, affecting daily activities, emotional well-being, and even professional performance. Sufferers may experience difficulty concentrating, sleeping disturbances, and heightened stress levels. The chronic nature of tinnitus leads many to seek relief through various means. Unfortunately, for some, the relentless presence of tinnitus may result in anxiety or depression. Thus, finding effective treatments is not just about silence—it's about restoring peace and normalcy to the lives of those affected.
Current Treatments for Tinnitus
Current treatments for tinnitus include sound therapy, cognitive-behavioral therapy, hearing aids, and medication to manage symptoms. These approaches aim to reduce the perception or distress associated with tinnitus but often do not provide a cure. For many, relief is fleeting and incomplete. The variability of tinnitus symptoms among individuals complicates the development of a one-size-fits-all solution, emphasizing the need for personalized and more effective treatment options.
Try this tonight at home…
Scientists have recently discovered an unusual technique that can reduce tinnitus…
This strange “hearing hack” is so powerful it does not take a lot of time, and works regardless of...
Introduction to OTO-313 and Its Potential
The Science Behind OTO-313
OTO-313 is a groundbreaking investigational therapy designed to target the root causes of tinnitus. It is a sustained-exposure formulation of gacyclidine, a non-competitive NMDA receptor antagonist. By modulating glutamatergic neurotransmission in the cochlea and central auditory pathways, OTO-313 aims to reduce aberrant neuronal activity responsible for the perception of tinnitus. This targeted approach offers hope for a more effective and long-lasting treatment compared to traditional methods.
How OTO-313 Differs from Other Treatments
Unlike conventional tinnitus treatments that primarily manage symptoms, OTO-313 seeks to alter the underlying neural mechanisms of tinnitus. Its unique formulation allows for direct administration to the inner ear, potentially leading to a more pronounced and durable therapeutic effect. By focusing on the neural activity linked to tinnitus, OTO-313 represents a novel approach that could revolutionize the management of this challenging condition.
The Evolution of OTO-313 Research
Early Preclinical Studies
Before advancing to human trials, OTO-313 underwent rigorous preclinical studies to assess its efficacy and safety. These early stages of research provided promising indications that OTO-313 could significantly diminish tinnitus-related activity in animal models. The results propelled the compound into clinical development, setting the stage for further investigation into its potential benefits for human patients.
This Quick Technique is Surprisingly Effective
This quickly applied Technique is Unusually Effective
Phase 1 Clinical Trial Results
The transition from animal models to human subjects began with Phase 1 clinical trials, which primarily focused on safety and tolerability. The outcomes were encouraging, as OTO-313 demonstrated a favorable safety profile with minimal side effects. These initial human trials laid a solid foundation for subsequent Phase 2 studies, aimed at evaluating the treatment's effectiveness in a larger tinnitus-afflicted population.
Progression to Phase 2 Trials
Building on the success of Phase 1, OTO-313 moved into Phase 2 clinical trials to explore its therapeutic potential more comprehensively. These trials were designed to assess the efficacy of OTO-313 in alleviating tinnitus symptoms over a more extended period. The advancement to this critical phase of research marked a significant milestone in the journey towards a potential new solution for tinnitus sufferers.
Insights from Phase 2 Clinical Trials of OTO-313
Trial Design and Objectives
Phase 2 clinical trials for OTO-313 employed a randomized, double-blind, placebo-controlled design to ensure the reliability of results. The primary objective was to determine the efficacy of OTO-313 in reducing the severity of tinnitus. Secondary objectives included evaluating the duration of relief, impact on quality of life, and any potential side effects. These trials are pivotal in ascertaining whether OTO-313 can fulfill the promise suggested by earlier research.
Scientist’s Discovery Quickly Addresses Hearing Loss…
Hundreds of thousands are already using this “weird hack”…
Efficacy of OTO-313 in Tinnitus Relief
Emerging data from Phase 2 trials has provided compelling evidence of OTO-313's efficacy in tinnitus relief. Participants reported a noticeable improvement in their symptoms, with some experiencing a significant reduction in the loudness and annoyance of tinnitus. These findings suggest that OTO-313 could represent a major breakthrough in the treatment of tinnitus, offering newfound hope to millions struggling with this condition.
Safety Profile and Tolerability
Alongside efficacy, the safety and tolerability of OTO-313 are of utmost importance. Phase 2 trials have continued to affirm the treatment's favorable safety profile observed in Phase 1. Reports indicate that OTO-313 is well-tolerated among participants, with no severe adverse events linked to the treatment. This reinforces the potential of OTO-313 as a safe and effective option for tinnitus management.
Patient Experiences with OTO-313
Personal Stories of Tinnitus Sufferers
The real-world impact of OTO-313 is best illustrated through the personal stories of tinnitus sufferers who have participated in clinical trials. These individuals often speak of the relentless nature of tinnitus and the ways it has disrupted their lives. For some trial participants, OTO-313 has brought a long-awaited reprieve, allowing them to enjoy moments of quietude and improved concentration, which they had not experienced in years.
Scientist’s Discovery Means a Lot for Hearing Loss…
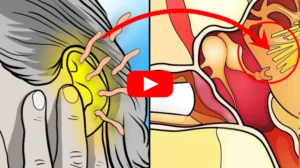
Thousands of people are already using this “strange hack”…
Reported Outcomes from OTO-313 Treatment
Reported outcomes from those who have received OTO-313 treatment are encouraging. While individual experiences vary, a common thread is the reported reduction in the perceived volume of tinnitus, leading to better sleep and reduced anxiety. These personal accounts contribute to a growing body of evidence supporting OTO-313's potential and underscore the importance of patient-centered outcomes in evaluating treatment success.
The Future of Tinnitus Treatment with OTO-313
Next Steps in OTO-313 Development
The journey of OTO-313 is far from over. As Phase 2 trials conclude, the next steps involve analyzing the data to refine dosing strategies and to plan for larger Phase 3 trials. These future studies will be critical in confirming OTO-313's efficacy and safety on a broader scale, which is necessary for regulatory approval and eventual market availability.
Potential for Combination Therapies
OTO-313's potential extends beyond standalone use. Researchers are also exploring its use in combination with other therapies, such as sound therapy or psychological interventions. This multimodal approach could provide a comprehensive treatment strategy addressing both the physiological and psychological aspects of tinnitus, catering to the diverse needs of the tinnitus population.
This ANCIENT HERB Might Bring Silence To Your Life
Reduce Ear Buzzing Using This Pinch Method
Implications for Personalized Tinnitus Management
The advent of OTO-313 may usher in a new era of personalized tinnitus management. By understanding individual differences in tinnitus etiology and response to treatment, clinicians could tailor therapies to better align with each patient's unique condition. Personalized management holds the promise of more effective and satisfying outcomes for those living with tinnitus.
Overcoming Challenges in Tinnitus Research
Technical and Scientific Hurdles
Tinnitus research is fraught with challenges, including the subjective nature of the condition and the lack of objective biomarkers. Overcoming these hurdles requires innovative research methodologies and ongoing scientific exploration. The development of OTO-313 reflects a commitment to addressing these complexities and advancing our understanding of tinnitus treatments.
Regulatory Considerations
Bringing a new treatment to market involves navigating a myriad of regulatory considerations. OTO-313 must undergo rigorous evaluation by health authorities to ensure its safety and efficacy. This process is crucial for protecting patients while also facilitating access to new therapies that can significantly improve their lives.
This ANCIENT HERB Might Bring Silence To Your Life
Reduce Ear Buzzing Using This Method
Ensuring Patient-Centered Research
At the heart of tinnitus research lies the imperative to maintain a patient-centered approach. By prioritizing the experiences and needs of individuals with tinnitus, researchers and clinicians can ensure that the development of treatments like OTO-313 truly benefits those they are meant to serve. It is through this lens that the most meaningful advancements in tinnitus relief will be realized.
Conclusion: The Prospects of OTO-313 in Tinnitus Relief
Summary of Key Research Findings
OTO-313 emerges as a promising candidate in the quest for tinnitus relief, with key research findings indicating its potential to significantly reduce the symptoms of this pervasive condition. The sustained exposure to gacyclidine, its favorable safety profile, and the positive patient outcomes reported from clinical trials all contribute to the optimism surrounding this novel treatment.
The Importance of Continued Research and Support
The advancement of OTO-313 highlights the importance of continued research and support in the field of audiology and neurology. As we gain a deeper understanding of tinnitus and the mechanisms underlying its treatment, we can offer more effective solutions to those who suffer from it. Supporting research initiatives will ensure that progress towards a future free from tinnitus continues unabated.
A Glimpse into a Future without Tinnitus
While the journey to a definitive cure for tinnitus is ongoing, the development of OTO-313 offers a glimpse into a future where this condition can be effectively managed or even eliminated. The prospects of OTO-313 provide hope and serve as a testament to the power of scientific innovation and dedication to improving the lives of those with hearing disorders. As research presses forward, we remain optimistic about the role OTO-313 may play in the future of tinnitus treatment.

Laura Henderson is a health enthusiast and has been interested in healthy and natural methods of eliminating tinnitus and restoring natural hearing for many years.